Im my "A-level" chemistry work on the benzene molecule my teacher mentioned the idea of anti-bonding orbitals. I would be interested to hear some kind of explanation about them purely for my own interest. thanks.
I assume you have searched our archives for "antibonding". The answers there will give some idea, but I happen to hold this discussion every year for my organic chemistry students, so I have all the diagrams available. As a matter of fact, I've written a number of MadSci answers, with graphics, on molecular orbitals and similar topics:
- What's up with the Lewis dot structure of sulfate? This answer is quite detailed, and includes some discussion of antibonding orbitals.
- Why don't two carbon atoms form a molecule with four covalent bonds?
- How are the AO's in IF7 selected to make a d3sp3 hybrid?
Ahem.
Anti-bonding molecular orbitals (MOs) result from the rules we use to construct MOs from atomic orbitals (AOs), the so-called Linear Combination of Atomic Orbitals or LCAO approach. MOs so constructed must follow two rules:
- Orbitals must combine according to their symmetry.
- For every AO that is used, you must get one MO back.
Let's use hydrogen (H2) as an example. Each hydrogen atom
contributes a 1s AO, which is a standing wave with no nodes. These can
combine in two ways:
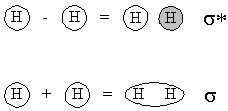
- Subtracting, so that the two 1s orbitals have the opposite phase.
- Adding, so that the two 1s orbitals have the same phase.
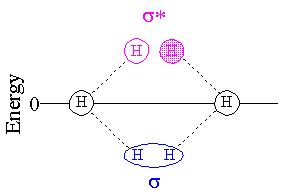
Antibonding MOs are normally higher in energy than bonding MOs, as shown in the diagram below. Since MOs are occupied two electrons at a time, and each hydrogen atom can contribute only one electron, only the s (bonding) MO is occupied and the H2 molecule is more stable than two separated H atoms.
|
 
|
In He2, both the bonding and antibonding orbitals are occupied and there is no net stabilization of the molecule versus the isolated atoms.
The reason an antibonding MO is antibonding, is because there is actually less wave density between the two nuclei than there would be if there was no bonding interaction at all. (I say "wave density" because if the orbital is not occupied, there will be nothing to say about electron density.) When an MO changes sign (from positive phase to negative phase--which I am representing as shaded/unshaded) between two atoms, it is said to be antibonding with respect to those atoms.
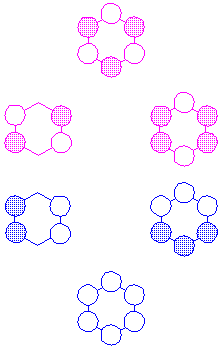
In molecules with several atoms, such as benzene, a particular MO may be bonding with respect to some adjacent pairs of atoms and antibonding with respect to other pairs. If the bonding interactions outnumber the antibonding interactions, the MO is said to be "bonding," while if the antibonding interactions outnumber the bonding interactions, the MO is said to be "antibonding." As an example, consider the p MOs of benzene:
|
|
||||||||
It can easily be seen that, while the lowest and highest MO are entirely bonding/antibonding, there are bonding interactions in the second row, right MO, which is nevertheless "antibonding;" and antibonding interactions in the third row, right MO, which is nevertheless "bonding."
Since each carbon atom contributes only one electron to the p-system of benzene, there are six p-electrons and only the three lowest-energy MOs--the bonding MOs--are filled.
Antibonding orbitals are important for explaining chemical reactions in terms of molecular-orbital theory; Hoffmann and Fukui shared the 1981 Nobel Prize in Chemistry for their development of qualitative MO explanations for chemical reactions.For more, see the classic text, The Importance of Antibonding Orbitals by Milton Orchin and H. H. Jaffe.
| Dan Berger |
| Bluffton College |
| http://www.bluffton.edu/~bergerd |