"Glubber" - what a great name! - is not a true chemical compound, but is a material who properties are dependent on intermolecular forces. The necessary ingredients are three: (1) Poly(vinyl alcohol). Poly(vinyl alcohol) is prepared by hydrolysis of poly(vinyl acetate) (1a) and usually will contain some acetate groups still; a low number of these is preferable, since they cannot bind strongly with the other ingredients. The higher the molecular weight (the greater the value of n in the formula) the greater the mechanical strength of the material.
(1)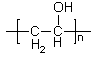
(1a)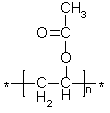
(2) Borax, Na2B4O7.10H2O. In water, this dissociates to give boric acid, B(OH)3. Above a pH of 9, this is present as the borate ion B(OH)4-, which forms strong hydrogen bonds with water and with other compounds with -OH groups.
(3) Water. You need lots of water to make poly(vinyl alcohol) slime, else you just have a powder.
Wood glue is essentially a dispersion of poly(vinyl alcohol) in water, so borax is the only additional ingredient required. The hydrogen bonds formed by borate between ľOH groups in adjacent polymer molecules are stronger than regular forces between molecules, but weaker than forces within a molecule.
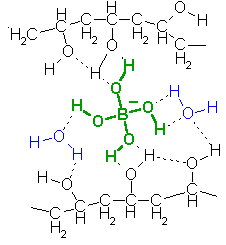
In the picture above the hydrogen bonds are drawn as dotted lines.
Rather than being permanent (in which case they would set the glubber into a rigid foam, like styrofoam), these bonds are continually breaking and re-forming. This takes time, and because the molecules are so very big and tangled up with each other, the time taken for them to move past one another is comparable to the lengths of time it takes for us humans to do something to the piece of glubber. So if we pull it slowly, the borate crosslinks have time to break and reform somewhere else (acting like bonds between molecules) and we can deform the glubber. But if we give it a rapid shock, the borate crosslinks will act more like bonds within a molecule, and the glubber will react elastically ľ the lengths of polymer between the borate crosslinks will stretch, but then spring back into their original positions.With the glue putty, I suspect the starch granules found in powdered starch are responsible. These are very much larger than the borate ions (microns across!) but their surfaces are covered with ľOH groups suitable for hydrogen bonding. Poly(vinyl alcohol) molecules could adsorb and desorb from the surface of these particles, giving crosslinks between polymers by a completely different mechanisms...
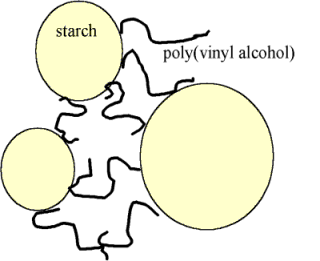
...but which could again break and re-form on the timescale necessary for glubberish behaviour.
This is based largely on an excellent article in the Journal of Chemical Education by Casassa, Sarquis, and Middleton (pp.57-61, volume 63, number 1, 1986), as well as on my own experiences with starch and with poly(vinyl alcohol)-based slime.