On a recent test of mine we had to draw NO2's lewis structure and say if it is polar and/or linear. I found drawings of the lewis structure in many places, but all seem to have different answers.
I am interested if it is linear or not (most lewis dot diagrams show the 1 electron on the nitrogen, but no bent shape), and if it is linear, whether its resonance structures combined, would make it non-polar.
Thanks, Ryan
Most Lewis structures don't properly indicate geometry (think about the Lewis structure for methane!). NO2 is, in fact, bent (the bond angle is 136°). The two most important resonance structures are shown below.
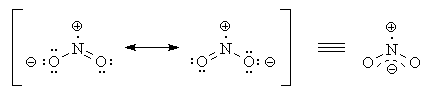
The way we would know, from the Lewis structure, that NO2 is bent is the fact that the nitrogen atom has three "groups" around it: two oxygen atoms and the unpaired electron. Atoms with three groups adopt a trigonal-planar configuration, with 120° bond angles.
The problem with this is that it is unclear whether unpaired electrons really take up all that much space. For example, methyl radical (CH3·) has four groups, but a trigonal planar geometry, while silyl radical (SiH3·) also has four groups, with a roughly tetrahedral geometry.
With this in mind, though, we can still explain the bent structure of NO2 via these additional resonance structures:
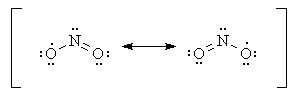
The Lewis structures in this answer were obtained by analysis of UHF/3-21G* calculated structures; the bond angle given is the calculated bond angle.
| Dan Berger |
| Bluffton College |
| http://cs.bluffton.edu/~berger |