Date: Wed Jan 29 19:15:14 2003
Posted By: Eric Maass, Director, semiconductors / communication products
Area of science: Physics
ID: 1042876533.Ph
Message:
Hello, Paul - sorry for the delay in replying to your question; I just
returned from a trip offshore. You've posed an interesting question,
and one that is in perhaps one of the more abstract areas of science,
so the difficulty in getting a conceptual picture of it despite your own
background and attempts by scientifically educated engineers on
your team is really not too surprising.
Anyway, the short answer is that the electrons pretty much originate
and stay within the conductor or are replenished with a source at the
more negative terminal, and no, the matter is not changed...but let's
start with the area of abstraction that makes this all confusing.
In a noble gas such as helium, each electron is pretty much tied to
one specific atom. If we pass electricity through a noble gas (and we
do with neon lights, for example), then the matter would be changed -
the gas in a neon light is ionized because some atoms have been
stripped of their electrons.
In simple compounds, the electrons become a little less locallized -
they can be shared between adjacent atoms in covalent bonds.
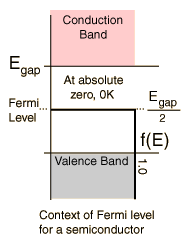
In crystalline, semiconducting materials such as silicon, the
electrons become even less locallized. The s and p orbitals and
such that we have come to know and love of the atoms in the crystal
lattice have combined together into two energy bands. There is one
energy band corresponding to the electrons that are tied to the
nucleii, called the valence band, and another energy band called the
conduction band corresponding to the relatively small number of
electrons that have enough energy (either just due to the temperature
or through doping methods) that they are available for conduction.
 Metals also have that crystalline structure, though they might not look
much like other crystals. Let me quote Dr. Richard Fitzpatrick,
Assistant Professor of Physics at University of Texas at Austin from his
lecture on his website: The conduction electrons in a metal are
non-localized (i.e., they are not tied to any particular atoms).
These non-localized electrons are what give metals many of their unusual properties,
including the reflectivity to light, electrical and thermal conductivity.
In metals, the conduction bands and valence bands basically
overlap. The electrons are sometimes referred to as an "electron
gas" - and some properties of this electron gas are modelled with
variations of the ideal gas law, as if they were a microscopically thin
layer of gas loosely adhering to the crystalline structure of the metal.
Not all of the electrons are actually conducting -
in copper at room temperature, less than 1% of electrons are
available to conduct electricity. This link calculates a value of 0.4%.
Let's come up with a few numbers for a specific example. For 15
gauge wire SWG or BWG (13 gauge AWG), with 2.63 square
millimeters for a cross sectional area, there are 2.2e23 total
electrons per meter. 0.4% of the electrons corresponds to 8.9e20
electrons per meter. To put it a bit into context, an Ampere is 6.28e18
electrons per second.
Let's come back to the electrons in motion. According to the
calculations
hyperphysics.phy-astr.gsu.edu/hbase/electric/ohmmic
here, the mean free path of electrons in copper is 3.9e-08 meter -
39 nanometers. Anyway, you asked, "But if electricity is electrons in
motion, where do they originate? " They are mostly already in the
metal, and the few electrons that come out of the more positive end of
a wire are replenished at the more negative end. At some point,
most circuits terminate at Ground. The earth itself, the Ground, can
be considered an infinite source of free electrons to replenish the
supply of electrons.
I hope this provides the explanation you were looking for. Please let
me know if you need any additional clarification.
And - my best wishes for Mendell Energy for continued success in
finding reserves of oil and gas - these efforts are increasingly
important to our future!
Best regards,
Eric Maass
Metals also have that crystalline structure, though they might not look
much like other crystals. Let me quote Dr. Richard Fitzpatrick,
Assistant Professor of Physics at University of Texas at Austin from his
lecture on his website: The conduction electrons in a metal are
non-localized (i.e., they are not tied to any particular atoms).
These non-localized electrons are what give metals many of their unusual properties,
including the reflectivity to light, electrical and thermal conductivity.
In metals, the conduction bands and valence bands basically
overlap. The electrons are sometimes referred to as an "electron
gas" - and some properties of this electron gas are modelled with
variations of the ideal gas law, as if they were a microscopically thin
layer of gas loosely adhering to the crystalline structure of the metal.
Not all of the electrons are actually conducting -
in copper at room temperature, less than 1% of electrons are
available to conduct electricity. This link calculates a value of 0.4%.
Let's come up with a few numbers for a specific example. For 15
gauge wire SWG or BWG (13 gauge AWG), with 2.63 square
millimeters for a cross sectional area, there are 2.2e23 total
electrons per meter. 0.4% of the electrons corresponds to 8.9e20
electrons per meter. To put it a bit into context, an Ampere is 6.28e18
electrons per second.
Let's come back to the electrons in motion. According to the
calculations
hyperphysics.phy-astr.gsu.edu/hbase/electric/ohmmic
here, the mean free path of electrons in copper is 3.9e-08 meter -
39 nanometers. Anyway, you asked, "But if electricity is electrons in
motion, where do they originate? " They are mostly already in the
metal, and the few electrons that come out of the more positive end of
a wire are replenished at the more negative end. At some point,
most circuits terminate at Ground. The earth itself, the Ground, can
be considered an infinite source of free electrons to replenish the
supply of electrons.
I hope this provides the explanation you were looking for. Please let
me know if you need any additional clarification.
And - my best wishes for Mendell Energy for continued success in
finding reserves of oil and gas - these efforts are increasingly
important to our future!
Best regards,
Eric Maass
Current Queue |
Current Queue for Physics |
Physics archives
Try the links in the MadSci Library for more information on Physics.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-2002. All rights reserved.
 Metals also have that crystalline structure, though they might not look
much like other crystals. Let me quote Dr. Richard Fitzpatrick,
Assistant Professor of Physics at University of Texas at Austin from his
lecture on his website: The conduction electrons in a metal are
non-localized (i.e., they are not tied to any particular atoms).
These non-localized electrons are what give metals many of their unusual properties,
including the reflectivity to light, electrical and thermal conductivity.
In metals, the conduction bands and valence bands basically
overlap. The electrons are sometimes referred to as an "electron
gas" - and some properties of this electron gas are modelled with
variations of the ideal gas law, as if they were a microscopically thin
layer of gas loosely adhering to the crystalline structure of the metal.
Not all of the electrons are actually conducting -
in copper at room temperature, less than 1% of electrons are
available to conduct electricity. This link calculates a value of 0.4%.
Let's come up with a few numbers for a specific example. For 15
gauge wire SWG or BWG (13 gauge AWG), with 2.63 square
millimeters for a cross sectional area, there are 2.2e23 total
electrons per meter. 0.4% of the electrons corresponds to 8.9e20
electrons per meter. To put it a bit into context, an Ampere is 6.28e18
electrons per second.
Let's come back to the electrons in motion. According to the
calculations
hyperphysics.phy-astr.gsu.edu/hbase/electric/ohmmic
here, the mean free path of electrons in copper is 3.9e-08 meter -
39 nanometers. Anyway, you asked, "But if electricity is electrons in
motion, where do they originate? " They are mostly already in the
metal, and the few electrons that come out of the more positive end of
a wire are replenished at the more negative end. At some point,
most circuits terminate at Ground. The earth itself, the Ground, can
be considered an infinite source of free electrons to replenish the
supply of electrons.
I hope this provides the explanation you were looking for. Please let
me know if you need any additional clarification.
And - my best wishes for Mendell Energy for continued success in
finding reserves of oil and gas - these efforts are increasingly
important to our future!
Best regards,
Eric Maass
Metals also have that crystalline structure, though they might not look
much like other crystals. Let me quote Dr. Richard Fitzpatrick,
Assistant Professor of Physics at University of Texas at Austin from his
lecture on his website: The conduction electrons in a metal are
non-localized (i.e., they are not tied to any particular atoms).
These non-localized electrons are what give metals many of their unusual properties,
including the reflectivity to light, electrical and thermal conductivity.
In metals, the conduction bands and valence bands basically
overlap. The electrons are sometimes referred to as an "electron
gas" - and some properties of this electron gas are modelled with
variations of the ideal gas law, as if they were a microscopically thin
layer of gas loosely adhering to the crystalline structure of the metal.
Not all of the electrons are actually conducting -
in copper at room temperature, less than 1% of electrons are
available to conduct electricity. This link calculates a value of 0.4%.
Let's come up with a few numbers for a specific example. For 15
gauge wire SWG or BWG (13 gauge AWG), with 2.63 square
millimeters for a cross sectional area, there are 2.2e23 total
electrons per meter. 0.4% of the electrons corresponds to 8.9e20
electrons per meter. To put it a bit into context, an Ampere is 6.28e18
electrons per second.
Let's come back to the electrons in motion. According to the
calculations
hyperphysics.phy-astr.gsu.edu/hbase/electric/ohmmic
here, the mean free path of electrons in copper is 3.9e-08 meter -
39 nanometers. Anyway, you asked, "But if electricity is electrons in
motion, where do they originate? " They are mostly already in the
metal, and the few electrons that come out of the more positive end of
a wire are replenished at the more negative end. At some point,
most circuits terminate at Ground. The earth itself, the Ground, can
be considered an infinite source of free electrons to replenish the
supply of electrons.
I hope this provides the explanation you were looking for. Please let
me know if you need any additional clarification.
And - my best wishes for Mendell Energy for continued success in
finding reserves of oil and gas - these efforts are increasingly
important to our future!
Best regards,
Eric Maass