| MadSci Network: Biochemistry |
Hi Tealah,
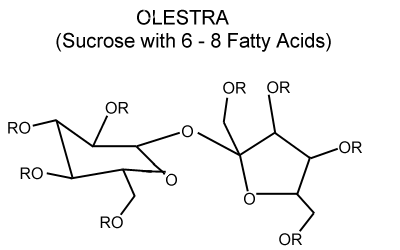
Olestra (aka Olean) is the popular name for a sucrose polymer (a Sucrose Octaester) first produced by Procter and Gamble in 1968 as a synthetic fat-substitute. Olestra consists of a molecule of sucrose (the di-saccharide consisting of glucose and fructose, also known as table sugar) attached to eight fatty acid molecules as described in the following figure:

The R groups in this figure are fatty acids, which can have a variety of chemical structures that (when saturated) can be written as CH3-(CH2)n-COO-.
The fats in our foods also contain fatty acids, but these are attached to a glycerol molecule, three at a time. The formula of glycerol is CH2OH-CHOH-CH2OH.
Because Olestra contains so many fatty acids, it can be used just like a real fat in cooking, and in fact, it tastes just like real fat. But because it does not occur in nature, we don't have any enzymes to digest it, and we can't use it as a food source. It just passes right through our digestive system. So, you won't "get fat" from Olestra.
However, the Olestra potato chips contain starch as well as Olestra, and starch is a carbohydrate that we can digest and metabolize. People tend to think of things like fats, proteins and carbohydrates as being completely different, but as far as your body is concerned, they are basically interchangeable -- it is a trivial matter for our cells to break down fats and make them into sugars, or to break down starches into sugars and then break those down and make them into fats. In fact, this is why we (animals) put on fat; we use it as a storage molecule for food that we have eaten, but which we don't need to use for energy. If you eat any sort of food in excess of your metabolic needs, fats, carbs or protein, your body can easily convert that food into fat.
This is done in the cell at the intersection of the fatty-acid metabolism pathway, the glucose metabolism (glycolysis) pathway and the amino-acid metabolism pathway, all of which lead to the molecule Acetyl-CoA. Your body makes a decision with what to do with Acetyl-CoA depending on its metabolic needs. If it has a lot of Acetyl-CoA, and it needs energy, it can use it to generate ATP by putting it into the tricarboxylic acid cycle (aka the citric acid cycle). If it has a lot of Acetyl-CoA, and it needs to make more glucose (perhaps to be stored as glycogen), it can use Acetyl-CoA in a process known as gluconeogenesis, or if it doesn't need energy and it doesn't need glucose, it can use it to make fatty acids, which are then stored as fat.
So, even though Olestra isn't digestible, you can still put on fat from eating too many Olestra potato chips.
You can find out more information about Acetyl-CoA metabolism in a college-level Biochemistry textbook such as Biochemsitry by Lubert Stryer.
I went to Procter and Gamble's Olestra web site to look for information on the development of Olestra, and found this reference:
Jandacek RJ (1989). The Development, Properties and Utilization of Olestra, a Nonabsorbable Fat Substitute. Proceedings of the 1989 Annual ACS Corporation Associates Symposium, 59-65.
Moderator's Note: While everything that I stated above is true for bacteria like E. coli, it is not true that humans can initiate gluconeogenesis with AcetylCoA. This is discussed in greater detail in this answer (1081350313.Bc). So, humans cannot make carbohydrates from fats.
Try the links in the MadSci Library for more information on Biochemistry.