| MadSci Network: Biochemistry |
Hi Narges. I'm sorry that it has taken so long to respond to your question, and I'm afraid that we can't really know what is going on on your end without knowing more about your ELISA-like assay, and in addition (as you will see below), it depends on what you mean by "base pairing."
5-Bromo-uracil (BrU) forms standard Watson-Crick (WC) base pairs with Adenine, and it can also form a base pair with Guanine, which is why some people use it for mutagenesis.
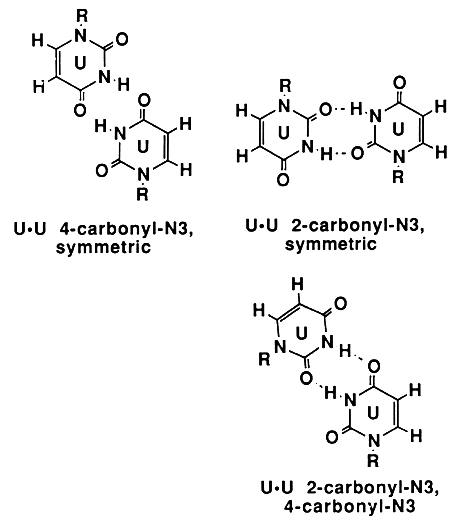
As you likely know, WC base pairs are defined by their hydrogen bonding geometry. The overall geometry of A:U, U:A, C:G, and G:C base pairs are pretty much identical, so that the nucleic acid helix remains symmetrical. However, there are numerous additional ways to make hydrogen bonds between bases. For example, A & U and G & C can form non-WC base pairs known as Hoogsteen base pairs that each involve two different pairs of hydrogen bonds. The Hoogsteen conformation of the A:U base pair is more stable than the WC form when the A and U are in solution as opposed to being in a polynucleotide. Tinoco (see below) has determined that there are 28 ways to pair bases together with two hydrogen bonds. Three of these are U:U base pairs, and these are shown in the figure below (taken without permission from "Ignacio Tinoco, Jr. in Gesteland, R. F. and Atkins, J. F. (1993) THE RNA WORLD. Cold Spring Harbor Laboratory Press"). In addition, you can find many more examples in the non-canonical base pair database.

So, it is quite possible that your 5BrU could form a non-WC base pair with Thymine (since Thymine is just 5-Methyl-Uracil), depending on how your assay works....
In addition, 5BrU is known to be extremely photolabile -- this means that it will form crosslinks with other molecules in the presence of light. Again, depending on how your assay works (if it involves some sort of stacking/intercolation of bases), you may be getting pyrimidine dimers between the 5BrU and the Thymine.
In RNA molecules, many structures can form using non-WC base pairing and other hydrogen bonds. One example is the so-called tetraloop structure. This is basically a really sharp bend where the RNA molecule folds back on itself. The structure of the actual turn involves 4 bases that make hydrogen bonding contacts with the phosphates in the backbone, and possibly with the 2' hydroxyl on the Ribose, in addition to each other. Since you specifically asked about DNA and not RNA, there is no 2' hydroxyl to make contacts with, but there is still the potential for hydrogen bonding with the phosphate in some wierd configuration.
So in conclusion, when you take nucleotides out of the context of double-stranded DNA, you can get all sorts of non-WC base pairs forming, and it is quite possible that your BrU could form hydrogen bonds with a Thymine base in your assay.
Here are some references if you want to do some more research into this topic, or if you are just curious.
Ignacio Tinoco, Jr. in Gesteland, R. F. and Atkins, J. F. (1993) THE RNA WORLD. Cold Spring Harbor Laboratory Press.
Jun Aishima, Rossitza K. Gitti, Joyce E. Noah, Hin Hark Gan, Tamar Schlick, and Cynthia Wolberger (2002) A Hoogsteen base pair embedded in undistorted B-DNA Nucleic Acids Res . December 1; 30 (23): 5244–5252.
Leontis NB, Stombaugh J, Westhof E. (2002) The non-Watson-Crick base pairs and their associated isostericity matrices. Nucleic Acids Res. Aug 15;30(16):3497-531.
Try the links in the MadSci Library for more information on Biochemistry.