Date: Sat May 29 20:04:03 2004
Posted By: Steve Mack, Post-doc/Fellow, Molecular and Cell Biology
Area of science: Biochemistry
ID: 1085836091.Bc
Message:
Thanks for another great question Jithesh.
So-called hydrophobic "bonds" are the result of the "hydrophobic effect",
which is driven by changes in entropy rather than enthalpy.
In solution, water molecules are free to rapidly exchange hydrogen bonds with their
neighbors, but because they cannot make hydrogen bonds with hydrophobic molecules,
those water molecules close to the surface of a hydrophobic molecule are constrained
in the number of neighbors with whom they can exchange hydrogen bonds. As a result, the
water molecules near the surface of hydrophobic molecules remain hydrogen bonded with
their neighbors much longer than they would have in solution (because they don't have any
other neighbors to exchange hydrogen bonds with), and hydrophobic molecules are said to
be surrounded in an ice-like "shell" of water molecules.
The number of water molecules in these "shells" is a function of the total surface area of the
hydrophobic molecules in the solution. 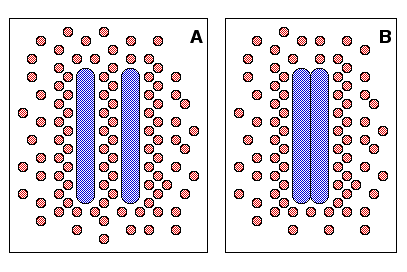 So, when hydrophobic molecules aggregate, the
total hydrophobic surface area is lower than when those molecules are separate, and the
number of water molecules not involved in these "shells" increases. This is demonstrated in the
figure to the right, where two large hydrophobic molecules are shown in blue, and the water
molecules are shown in red. You can see the "frozen" water molecules "trapped" between the two
hydrophobic molecules in figure A. In figure B, where the two hydrophobic molecules are together
in an aggregate, those water molecules that were "trapped" are free to join the rest of the water
molecules in solution. So, when hydrophobic molecules aggregate, the number of water molecules
in ice-like "shells" is minimized and entropy in that solution is maximized (assuming that the
temperature and the molecules themselves stay the same).
So, when hydrophobic molecules aggregate, the
total hydrophobic surface area is lower than when those molecules are separate, and the
number of water molecules not involved in these "shells" increases. This is demonstrated in the
figure to the right, where two large hydrophobic molecules are shown in blue, and the water
molecules are shown in red. You can see the "frozen" water molecules "trapped" between the two
hydrophobic molecules in figure A. In figure B, where the two hydrophobic molecules are together
in an aggregate, those water molecules that were "trapped" are free to join the rest of the water
molecules in solution. So, when hydrophobic molecules aggregate, the number of water molecules
in ice-like "shells" is minimized and entropy in that solution is maximized (assuming that the
temperature and the molecules themselves stay the same).
With this in mind, we can calculate the change in the Gibbs free energy of such a system,
which is expressed as
G = H-(T*S)
, where G is the Gibbs free energy, H is
the enthalpy, T is the temperature, and S is the entropy. A negative G indicates
that a reaction will proceed, and you can see that as G decreases as S increases, with no
change necessary to H. Now, the second law of thermodynamics tells us that entropy tends
to increase, which explains why the hydrophobic effect results in the spontaneous formation
of so-called hydrophobic bonds.
So now, I hope you can see why changes in enthalpy are not necessary for the formation of so-
called hydrophobic bonds.
You can find more information about the hydrophobic effect by reading
The
hydrophobic effect: formation of micelles and biological membranes, second edition by
Charles Tanford. (Krieger publishing company, Malabar, Florida, August 1991)
Current Queue |
Current Queue for Biochemistry |
Biochemistry archives
Try the links in the MadSci Library for more information on Biochemistry.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-2003. All rights reserved.
 So, when hydrophobic molecules aggregate, the
total hydrophobic surface area is lower than when those molecules are separate, and the
number of water molecules not involved in these "shells" increases. This is demonstrated in the
figure to the right, where two large hydrophobic molecules are shown in blue, and the water
molecules are shown in red. You can see the "frozen" water molecules "trapped" between the two
hydrophobic molecules in figure A. In figure B, where the two hydrophobic molecules are together
in an aggregate, those water molecules that were "trapped" are free to join the rest of the water
molecules in solution. So, when hydrophobic molecules aggregate, the number of water molecules
in ice-like "shells" is minimized and entropy in that solution is maximized (assuming that the
temperature and the molecules themselves stay the same).
So, when hydrophobic molecules aggregate, the
total hydrophobic surface area is lower than when those molecules are separate, and the
number of water molecules not involved in these "shells" increases. This is demonstrated in the
figure to the right, where two large hydrophobic molecules are shown in blue, and the water
molecules are shown in red. You can see the "frozen" water molecules "trapped" between the two
hydrophobic molecules in figure A. In figure B, where the two hydrophobic molecules are together
in an aggregate, those water molecules that were "trapped" are free to join the rest of the water
molecules in solution. So, when hydrophobic molecules aggregate, the number of water molecules
in ice-like "shells" is minimized and entropy in that solution is maximized (assuming that the
temperature and the molecules themselves stay the same).