Date: Fri Nov 12 12:05:09 2004
Posted By: Joseph Weeks, Engineer
Area of science: Physics
ID: 1099354239.Ph
Message:
Coffee is a beverage loved by many, but only if it is hot. Your question
is very interesting and as far as I can tell, no one has provided any data
on the effects of holding a cup of coffee.
There has been a lot of discussion about whether coffee stays hotter if the
cream and sugar are added when the coffee is served or if one delays adding
it until just before it is consumed. It turns out that the work of Cornell
University students Darwin Novak and Robert Seidel in 1958 clearly
established that your coffee stays hotter if the cream and sugar are added
immediately, rather than waiting (see
http://www.eweek.org/site/News/Features/coffee.shtml).
Most people who analyze the problem of when to add cream simply look
at the problem as one of conduction and ignore the convective heat losses
from the surface of the coffee. Put simply, heat is lost by evaporation much
more quickly from a hot liquid surface than from a cooler surface.
So, in considering how to answer your question, there are a whole bunch of
variables, and calculating a result based upon those assumptions seems
unsatisfying. So, instead, I decided to run a couple of experiments and
actually measure the results. You can argue over opinions for days; it is
hard to argue with data.
For my experiment, I used a genuine McDonald’s Styrofoam coffee cup with
the included lid as the container. For our test, I used water instead of
coffee, since coffee is mostly water. The weight of the empty cup was
7.008 grams; without the lid, the cup weighed 4.406 grams. I filled the
cup with deionized water and weighed the cup. I then heated the cup for 3
minutes in a microwave oven. The water was boiling at the time I removed
it from the oven. I quickly weighed the cup (having tared out the weight
of the cup), and then placed two small Type K thermocouples in the water in
the cup. The temperature decrease over time was measured using a PC and
data acquisition system. Room temperature was monitored with two other
thermocouples and remained between 18 and 20C throughout the experiment.
Typical brewing temperatures according to Novak and Seidel for coffee is
85C (185F) and drinking temperature is 62C(143F). I ran three experiments.
In the first experiments, the coffee cup containing the water sat on top
of a book in a room with stagnant air flow with the lid on. In the second
experiment, the cup sat in the same room, on the same book, but with the
lid off of the cup. In the third experiment, I held the coffee cup in my
hands for the first 40 minutes, alternating the cup from one hand to the
other occasionally, again with a lid on the cup.
In the first experiment the initial water weight after heating was 285.6g
after cooling the weight was 283.857 grams.
In the second experiment without the lid, the initial weight was 295.08
grams. When it was cooled, the weight was 277.26 grams.
In the third experiment where I held on to the coffee cup, the initial
weight of water was 285.72 grams. Final weight after cooling was 283.899.
The results of my experiment are shown in the following Figure:
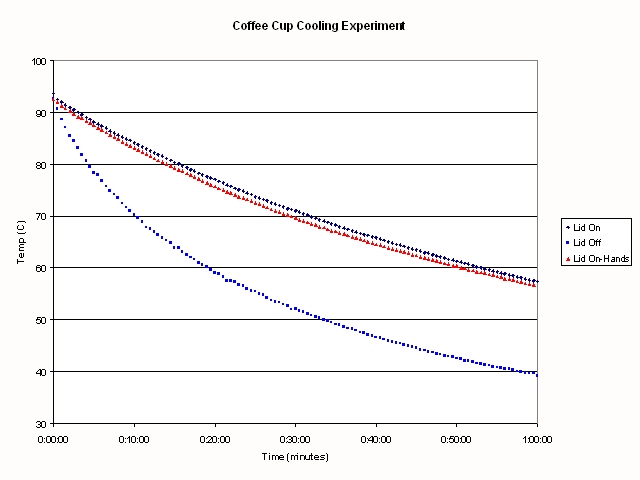 .
The first thing that is obvious is that there is a huge difference in
cooling rate depending upon whether there is a lid on your coffee cup or
not. The time for the water to decrease in temperature from 85C to 62C is
15 minutes if the lid is off the coffee. When the lid is on the coffee, it took
39.5 minutes to cool from brewing to drinking temperature if I wasn't
holding the cup, and 38 minutes if I was holding the cup.
Although it appears that holding the cup increases the rate that the cup
loses temperature, I wanted to take a closer look at the data. This second
Figure:
.
The first thing that is obvious is that there is a huge difference in
cooling rate depending upon whether there is a lid on your coffee cup or
not. The time for the water to decrease in temperature from 85C to 62C is
15 minutes if the lid is off the coffee. When the lid is on the coffee, it took
39.5 minutes to cool from brewing to drinking temperature if I wasn't
holding the cup, and 38 minutes if I was holding the cup.
Although it appears that holding the cup increases the rate that the cup
loses temperature, I wanted to take a closer look at the data. This second
Figure:
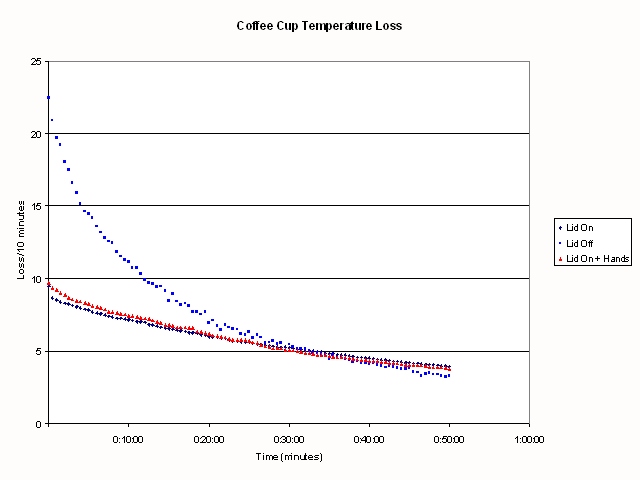 shows the number of degrees
temperature loss from each cup in ten minutes. If you carefully compare
the curves where I am holding the cup versus allowing the cup to stand in
still air, you can see that for the first 30 minutes or so, the rate of
heat loss from holding the cup is slightly greater than the standing cup.
You can also see from this Figure some bobbles in the graph when I changed
from one hand to the other, and when I got bored of holding the cup and sat
it down on the book (about 30-35 minutes into the experiment).
So, you might ask, why didn't I hold the cup while the lid was off? As you
can see from the data, the heat loss by evaporation of the open cup is
huge. Over a period of about 80 minutes, we lost 17.82 grams of water by
evaporation, whereas the closed cups lost about 1.8 grams to evaporation
and whatever quantity of water stuck to the underside of the lid. At 540
calories per gram of water evaporated, that is a loss of 9623 calories!
If I am holding the cup, my breath over the top of the cup could easily
increase the rate of evaporation and temperature loss, completely masking
the effects of holding the cup.
If you want your coffee or hot chocolate to stay hot, keep a lid on the
liquid. If we ran the same experiment with a glass cup, it is likely that
holding the cup in your hands would again increase the rate of heat loss,
however, a cup filled with 85C water would likely burn your hand at the
same time (because of the higher rate of heat transfer of glass compared
with styrofoam).
So, in summary, a cup of water with a lid on sitting on a book lost an
average of 36.07C in one hour, compared to a loss of 36.54C when the cup
was held in my hand. Without the lid, the cup lost 53.35C in an hour.
(These numbers were obtained by averaging the two thermocouple readings).
So, it appears that holding a coffee cup increases the rate of heat loss,
but not by much. It is a small price to pay for the pleasure of having
warmer hands on a cold fall or winter morning, plus you are utilizing heat
that would otherwise be lost to the environment.
And never let it be said that we Mad Scientists don't occasionally put a
lot of time and effort into answering your questions. Thanks for asking.
shows the number of degrees
temperature loss from each cup in ten minutes. If you carefully compare
the curves where I am holding the cup versus allowing the cup to stand in
still air, you can see that for the first 30 minutes or so, the rate of
heat loss from holding the cup is slightly greater than the standing cup.
You can also see from this Figure some bobbles in the graph when I changed
from one hand to the other, and when I got bored of holding the cup and sat
it down on the book (about 30-35 minutes into the experiment).
So, you might ask, why didn't I hold the cup while the lid was off? As you
can see from the data, the heat loss by evaporation of the open cup is
huge. Over a period of about 80 minutes, we lost 17.82 grams of water by
evaporation, whereas the closed cups lost about 1.8 grams to evaporation
and whatever quantity of water stuck to the underside of the lid. At 540
calories per gram of water evaporated, that is a loss of 9623 calories!
If I am holding the cup, my breath over the top of the cup could easily
increase the rate of evaporation and temperature loss, completely masking
the effects of holding the cup.
If you want your coffee or hot chocolate to stay hot, keep a lid on the
liquid. If we ran the same experiment with a glass cup, it is likely that
holding the cup in your hands would again increase the rate of heat loss,
however, a cup filled with 85C water would likely burn your hand at the
same time (because of the higher rate of heat transfer of glass compared
with styrofoam).
So, in summary, a cup of water with a lid on sitting on a book lost an
average of 36.07C in one hour, compared to a loss of 36.54C when the cup
was held in my hand. Without the lid, the cup lost 53.35C in an hour.
(These numbers were obtained by averaging the two thermocouple readings).
So, it appears that holding a coffee cup increases the rate of heat loss,
but not by much. It is a small price to pay for the pleasure of having
warmer hands on a cold fall or winter morning, plus you are utilizing heat
that would otherwise be lost to the environment.
And never let it be said that we Mad Scientists don't occasionally put a
lot of time and effort into answering your questions. Thanks for asking.
Current Queue |
Current Queue for Physics |
Physics archives
Try the links in the MadSci Library for more information on Physics.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@madsci.org
© 1995-2003. All rights reserved.
 .
The first thing that is obvious is that there is a huge difference in
cooling rate depending upon whether there is a lid on your coffee cup or
not. The time for the water to decrease in temperature from 85C to 62C is
15 minutes if the lid is off the coffee. When the lid is on the coffee, it took
39.5 minutes to cool from brewing to drinking temperature if I wasn't
holding the cup, and 38 minutes if I was holding the cup.
Although it appears that holding the cup increases the rate that the cup
loses temperature, I wanted to take a closer look at the data. This second
Figure:
.
The first thing that is obvious is that there is a huge difference in
cooling rate depending upon whether there is a lid on your coffee cup or
not. The time for the water to decrease in temperature from 85C to 62C is
15 minutes if the lid is off the coffee. When the lid is on the coffee, it took
39.5 minutes to cool from brewing to drinking temperature if I wasn't
holding the cup, and 38 minutes if I was holding the cup.
Although it appears that holding the cup increases the rate that the cup
loses temperature, I wanted to take a closer look at the data. This second
Figure:
 shows the number of degrees
temperature loss from each cup in ten minutes. If you carefully compare
the curves where I am holding the cup versus allowing the cup to stand in
still air, you can see that for the first 30 minutes or so, the rate of
heat loss from holding the cup is slightly greater than the standing cup.
You can also see from this Figure some bobbles in the graph when I changed
from one hand to the other, and when I got bored of holding the cup and sat
it down on the book (about 30-35 minutes into the experiment).
So, you might ask, why didn't I hold the cup while the lid was off? As you
can see from the data, the heat loss by evaporation of the open cup is
huge. Over a period of about 80 minutes, we lost 17.82 grams of water by
evaporation, whereas the closed cups lost about 1.8 grams to evaporation
and whatever quantity of water stuck to the underside of the lid. At 540
calories per gram of water evaporated, that is a loss of 9623 calories!
If I am holding the cup, my breath over the top of the cup could easily
increase the rate of evaporation and temperature loss, completely masking
the effects of holding the cup.
If you want your coffee or hot chocolate to stay hot, keep a lid on the
liquid. If we ran the same experiment with a glass cup, it is likely that
holding the cup in your hands would again increase the rate of heat loss,
however, a cup filled with 85C water would likely burn your hand at the
same time (because of the higher rate of heat transfer of glass compared
with styrofoam).
So, in summary, a cup of water with a lid on sitting on a book lost an
average of 36.07C in one hour, compared to a loss of 36.54C when the cup
was held in my hand. Without the lid, the cup lost 53.35C in an hour.
(These numbers were obtained by averaging the two thermocouple readings).
So, it appears that holding a coffee cup increases the rate of heat loss,
but not by much. It is a small price to pay for the pleasure of having
warmer hands on a cold fall or winter morning, plus you are utilizing heat
that would otherwise be lost to the environment.
And never let it be said that we Mad Scientists don't occasionally put a
lot of time and effort into answering your questions. Thanks for asking.
shows the number of degrees
temperature loss from each cup in ten minutes. If you carefully compare
the curves where I am holding the cup versus allowing the cup to stand in
still air, you can see that for the first 30 minutes or so, the rate of
heat loss from holding the cup is slightly greater than the standing cup.
You can also see from this Figure some bobbles in the graph when I changed
from one hand to the other, and when I got bored of holding the cup and sat
it down on the book (about 30-35 minutes into the experiment).
So, you might ask, why didn't I hold the cup while the lid was off? As you
can see from the data, the heat loss by evaporation of the open cup is
huge. Over a period of about 80 minutes, we lost 17.82 grams of water by
evaporation, whereas the closed cups lost about 1.8 grams to evaporation
and whatever quantity of water stuck to the underside of the lid. At 540
calories per gram of water evaporated, that is a loss of 9623 calories!
If I am holding the cup, my breath over the top of the cup could easily
increase the rate of evaporation and temperature loss, completely masking
the effects of holding the cup.
If you want your coffee or hot chocolate to stay hot, keep a lid on the
liquid. If we ran the same experiment with a glass cup, it is likely that
holding the cup in your hands would again increase the rate of heat loss,
however, a cup filled with 85C water would likely burn your hand at the
same time (because of the higher rate of heat transfer of glass compared
with styrofoam).
So, in summary, a cup of water with a lid on sitting on a book lost an
average of 36.07C in one hour, compared to a loss of 36.54C when the cup
was held in my hand. Without the lid, the cup lost 53.35C in an hour.
(These numbers were obtained by averaging the two thermocouple readings).
So, it appears that holding a coffee cup increases the rate of heat loss,
but not by much. It is a small price to pay for the pleasure of having
warmer hands on a cold fall or winter morning, plus you are utilizing heat
that would otherwise be lost to the environment.
And never let it be said that we Mad Scientists don't occasionally put a
lot of time and effort into answering your questions. Thanks for asking.