| MadSci Network: Physics |
What a great question. Determining why something breaks and how it breaks is part of the discipline of Fracture Mechanics. And analyzing the fracture surface can often tell a great deal about the material itself, as well as the mechanism by which it failed.
First of all, solid materials can be divided into metals and non-metals. One of the characteristics of metals is ductility; the ability to bend without breaking. Because the atoms in a metal are essentially twin sisters of each other, it is possible for metal atoms to shift position without breaking any chemical bonds. The atoms in non-metals (like glass) cannot shift positions without actually breaking chemical bonds, therefore non-metals tend to not bend rather than break when sufficient force is applied.
Non-metallic solid materials can be divided into two general classifications; crystalline materials and amorphous materials. Crystalline materials consist of ordered arrangements of molecules. There are generally planes in crystalline materials that have weaker chemical bonds than in other directions. Therefore, when you break a crystalline material, it will often produce flat regions corresponding to one of these cleavage planes. In fact, characteristic cleavage planes or crystal shapes are one of the better ways of recognizing different crystals. You can read a bit more about crystalline materials and cleavage at the University of Texas.
Glass is one of a group of materials that is able to solidify without assuming any particular regular crystalline structure. Therefore, there are no cleavage lines, no regular structure. Therefore, when glass fractures, the fracture line follows no specific direction but is free to move randomly through the material, responding only to the forces causing the fracture. There are many other amorphous solids that behave the same was as glass. Hard candy, particularly when it is clear, is an amorphous solid and fractures in the same way as glass. The type of fracture that is produced in glass will depend upon the type of forces applied to the glass, as well as the presence of flaws or “stress risers” that may be present. The mathematical treatment of forces surrounding a crack can be found in this tutorial on fracture mechanics from Duke University.
The mathematics of forces at a crack tip become very complex, involving partial differential equations. A few principles, however, can be easily understood. First, the sharper the tip of a crack is, the more force that will be concentrated at that point. Therefore, if a crack or flaw starts growing in a piece of glass, unless the forces are very low, the crack will continue to grow until the crack reaches an edge (or completes it’s path). If you think about what is happening on an atomic level when something breaks, if a crack is sharp enough, you essentially have all the external force concentrated between two atoms. If you have enough force to break that single atomic bond, then the crack can move to the next two atoms and the crack will grow through the material.
Of course sometimes there is a flaw in the material, or sometimes a different atom than its neighbors that has a different bond energy that may cause the crack to move toward that atom or away from that atom, producing some of the random crack growth that we see. The principle of using stress risers is used for cutting glass. A sharp tool is used to produce a scratch or flaw in the surface of the glass. Then, when bending forces are applied to the piece of glass, the force concentrates at the scratch, and fracture tends to occur at the scratch. If, however, two scratches are placed side by side (if more than one pass is made with the glass cutter), the crack may jump from one scratch to the other, and may often move randomly through the glass. That is the reason that when one cuts glass, you make one and only one pass with the glass cutter.
When cutting glass, the other requirement is to apply the right type of force so the glass breaks in the direction that you want. After scratching the glass where you want it to break, the piece of glass is placed on the edge of a table, with the scratch along the edge. When the unsupported side of the glass is pushed down, the top surface of the glass is put in tension, while the bottom surface is placed in compression. Like most ceramic materials, glass is weaker in tension rather than compression, so the fracture starts at the top surface, hopefully along the stress riser that we have supplied with the glass cutter.
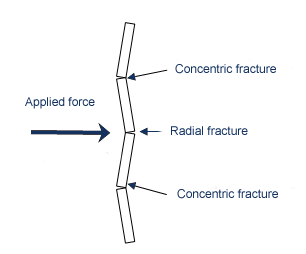
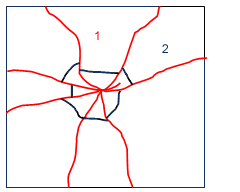
Without using a glass cutter, if uniform bending forces were applied to the piece of glass, it would still break generally along the edge of the table into two pieces. The energy required to keep a crack moving through glass is very small, since the cracks in glass are so fine and can concentrate so much force at the tip of the crack. Therefore, if you can cause several cracks to form in a piece of glass at the same time (by throwing a ball through a window for example), all of the cracks may have enough energy to move from their point of initiation until they reach a free surface. In the case of the ball, it is attempting to bend the glass more or less uniformly in all directions, therefore the glass tends to respond by producing a series of radial cracks that form from the point of impact. Since it takes very little energy to keep a crack moving through glass, the glass tends to fracture into concentric circles near the point of impact and a series of long tapered strips farther away from the point of impact. The type of cracks that result from impact are illustrated by these drawings, which were taken from this site on forensics, as a piece of glass responds to the forces of impact:


A lovely photo illustrating a glass fracture is shown here. You can see the concentric fractures near the point of impact, and the radial fractures which extend away from the point of impact.
 Fracture surfaces tend to be sharp. Since the fracture involves the
breaking of chemical bonds and the separation of atoms that were, until
recently, next to each other, the edge of a fracture surface consists of
atoms that theoretically come to a single point. Thus the edge of a cut
glass is very sharp, even if the angle on the edge is 90 degrees; it is the
sharpness or abruptness of the edge that causes it to be so
dangerous.
Fracture surfaces tend to be sharp. Since the fracture involves the
breaking of chemical bonds and the separation of atoms that were, until
recently, next to each other, the edge of a fracture surface consists of
atoms that theoretically come to a single point. Thus the edge of a cut
glass is very sharp, even if the angle on the edge is 90 degrees; it is the
sharpness or abruptness of the edge that causes it to be so
dangerous.
The fact that very little energy is needed to cause a crack to propagate is used to produce tempered or “safety” glass. Normal glass is cooled very slowly, or annealed to remove any internal stresses. Like all materials, glass contracts as it cools. Tempered glass is produced by heating a sheet of glass to high temperature, followed by blasting the outside surface blasted with cold air, while the interior of the glass is still hot. The outside surface becomes cold and rigid, while the inside of the glass is still relatively soft. As the interior cools, it places the outer surface in compression, while the interior of the glass is in tension. Because the outside surface is in compression, generally tempered glass is able to resist much larger forces without breaking than regular glass, since it takes a larger force to put the outside surface in tension. When tempered glass breaks, however, the internal forces of tension and compression within the glass are high enough that it tends to shatter in all directions simultaneously. So tempered glass tends to not produce the large, sharp pieces of glass that cause injury, instead producing a large number of very small pieces. It is simply a result of cracks moving through glass in all directions at the same time.
This effect was used as a party trick in the 1640's by Prince Rupert of Bavaria, in which a drop of glass could be hit with a hammer without breaking, however if the end of the tear-drop shape was snapped off, the drop would turn into powder. You can see a video of the technique at the Museum of Glass. I hope that provides you with a little insight into why glass breaks the way it does.
Try the links in the MadSci Library for more information on Physics.