| MadSci Network: Physics |
Your question doesn't give enough information to really understand what you are talking about, so I will have to do a bit of guessing. There are two types of bubbles; those that form within a liquid (such as when gas escapes from a carbonated drink, and those that are formed by blowing on a liquid film of "bubble solution." In either case, the interaction between air and liquid is determined by surface chemistry and surfactants.
In the first case, let's imagine you are a water molecule, surrounded by all sorts of other water molecules. There are forces between each molecule are very strong because they are based upon electrical charges. A water molecule has a negative end (the oxygen atom) and a positive end (the hydrogen atoms). These negative and positive charges attract nearby water molecules, making a dense liquid that has a remarkably high boiling point.
When you introduce a bubble of gas into water, you are forcing molecules at the water/gas surface to be surrounded by, and attracted to other water molecules on all sides except at the surface. Because there is nothing pulling on the water molecule from the gas surface, the water tries to pull away from the gas surface. This pulling force is referred to as surface tension. Because of surface tension, you can fill a glass of water slightly above the rim of the glass; the surface tension keeps the water from spilling over the side. A paper clip can sometimes float on the surface of water, and some insects can walk on water because of surface tension.
When a bubble forms inside a glass of water, the surface tension of the water surrounding the bubble tries to make the bubble as small as possible, so the bubble is in the form of a sphere (you have never seen a bubble look like a cube or a pyramid because each requires more surface area than a sphere). When the bubble floats to the surface of the water, surface tension pulls the water away from the bubble as fast a possible, causing the bubble to break and disappear.
Bubbles that are made by blowing a liquid film into a bubble are another example of surface tension and surface chemistry. Surfactant molecules change the surface tension or surface chemistry of liquids.
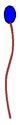
A surfactant molecule might look something like this, where one end of the molecule (the blue bulb) looks like a polar water molecule, and the other end of the molecule looks like a non-polar oil or hydrocarbon molecule. If you get a bunch of surfactant molecules together, they try and align with each other like this to form a film.
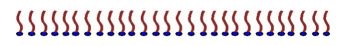
The polar ends of the surfactant molecules try to stick together, and the non-polar hydrocarbon ends of the molecule also try to stick together. If you put some of this surfactant molecule on top of water, it forms a film, as illustrated by this drawing.

The water molecules near the surfactant act as if they are surrounded by water molecules on all sides. If you have two layers of surfactant, you can trap a layer of water between the layers. Because of the strong attraction between water molecules, you end up with a liquid film that is quite strong, which is what you have in a soap bubble.
If we go back to our example of a gas bubble forming in you drink, the reason that the bubble normally disappears as soon as it reaches the surface is because there is nothing at the surface to stablize the thin liquid film of water surrounding the bubble. If you add some dishwashing detergent to your glass of carbonated drink, the bubbles will no longer disappear when they reach the surface, but instead will form a quite stable foam (of course, don't drink your carbonated drink with soap in it).
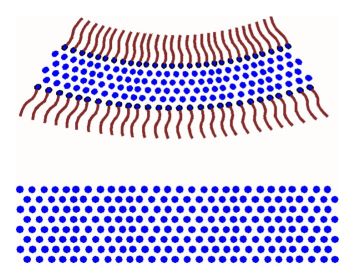
If you blow a bubble over a liquid surface, as the bubble settles onto the surface, suddenly the non-polar ends of the surfactant molecules come into contact with a polar surface, and the surfactant film along the bottom of the bubble breaks. Suddenly what had been a perfectly round bubble is now a hemisphere.
On the other hand, if you have a layer of surfactant on top of the water, as illustrated by the following image, you tend to have a more stable boundary between the bubble and the surface of the water.
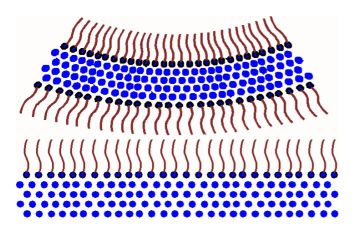
Surfactants have another very important function besides stabalizing bubbles. Surfactants allow water to surround and remove dirt from you hands and clothes. I hope that gives you a little insight into the science of surfactants and surface chemistry and hopefully answers the question you had in mind.
Try the links in the MadSci Library for more information on Physics.