Date: Wed Feb 24 02:26:13 1999
Posted By: Sven Nottebaum, Undergraduate, Molecular Biology, Theodor-Heuss-Gymnasium
Area of science: Molecular Biology
ID: 919562469.Mb
Message:
Dear Emily,
regarding why ethidium bromide is such a toxic mutagen, I'd like to tell
you first that although I'm quite new at this organization, I'll try and
help you in understanding your problem.
Attached to this response you'll find some descriptions about ethidium and
its nature as an intercalator. Intercalators, just as described, are
charged compounds that insert themselves between base pairs.
I hope you have already covered DNA REPLICATION and TRANSCRIPTION AND
TRANSLATION.For it is the crucial basis for understanding the effects of
ethidium bromide on the cell as a whole. As you know the cell is depended
on separating its DNA strands for replication and transcription in order
for DNA polymerase or RNA polymerase to copy the genetic information
respectively.
BUT if the cell was exposed to ethidium bromide it would incorporate itself
between the base pairs of the DNA and thus prevent such mechanisms of
replication or further protein synthesis. In that way it would behave
toxic, as the cell would soon die because it 1. CAN'T replicate anymore and
2. Because no further proteins that would enable the cell to survive are
synthesized.
Nevertheless, due its good fluorescence characteristics and the fact that
it binds to DNA, ethidium bromide is being used frequently in molecular
biological and biotechnical research. However, as you just stated because
it is such a toxic mutagen people are trying to substitute ethidium bromide
by other compounds that have the same fluorescence abilities and are less
toxic.
As I can think as your definition of a "toxic mutagen", is that as a
mutagen it effects the basis for heredity- the DNA of a cell. And it is so
"toxic" because it prevents the cell from accessing its genome to carry out
its necessary protein synthesis to survive.
I hope to have answered your question sufficiently and I'd like to mention
that I strongly encourage YOU and your peers to keep asking these kind of
questions. They should contribute to your all's understanding of the field
of molecular biology, I hope, and that's what we would like to achieve.
If you should have any further questions, you can also mail me directly at:
snottebaum@hotmail.com
I'd be glad to answer every question you had for me.
Sincerely,
Sven Nottebaum
Pictures:
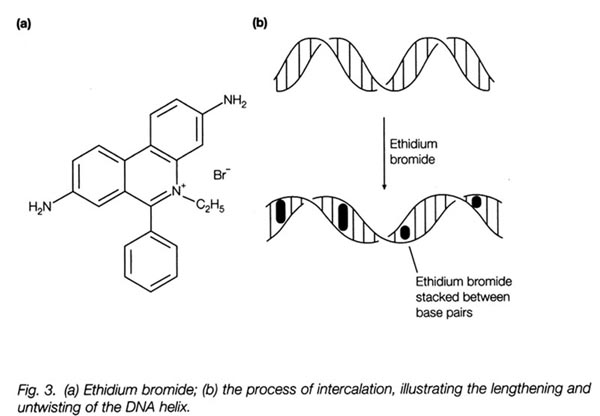
1. This shows you the structure of ethidium bromide and how it incorporates
itself between base pairs of the DNA
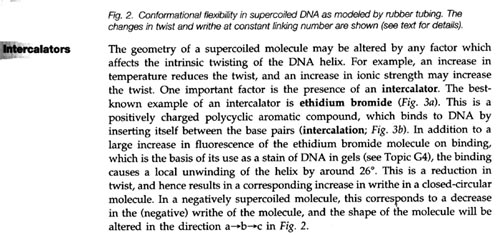
 2. This is a definition of intercalors ( just as ethidium bromide) from a
very interesting textbook, dealing with Molecular Biology. If you should
ever think about produding a bit further into this field, I highly
recommend this book. It's called: "Molecular Biology" and comes from the -
Instant Notes Series.(Instant notes in Molecular Biology; by Turner,
McLennan, Bates and White)
2. This is a definition of intercalors ( just as ethidium bromide) from a
very interesting textbook, dealing with Molecular Biology. If you should
ever think about produding a bit further into this field, I highly
recommend this book. It's called: "Molecular Biology" and comes from the -
Instant Notes Series.(Instant notes in Molecular Biology; by Turner,
McLennan, Bates and White)
Current Queue |
Current Queue for Molecular Biology |
Molecular Biology archives
Try the links in the MadSci Library for more information on Molecular Biology.
MadSci Home | Information |
Search |
Random Knowledge Generator |
MadSci Archives |
Mad Library | MAD Labs |
MAD FAQs |
Ask a ? |
Join Us! |
Help Support MadSci
MadSci Network,
webadmin@www.madsci.org
© 1995-1999. All rights reserved.
 2. This is a definition of intercalors ( just as ethidium bromide) from a
very interesting textbook, dealing with Molecular Biology. If you should
ever think about produding a bit further into this field, I highly
recommend this book. It's called: "Molecular Biology" and comes from the -
Instant Notes Series.(Instant notes in Molecular Biology; by Turner,
McLennan, Bates and White)
2. This is a definition of intercalors ( just as ethidium bromide) from a
very interesting textbook, dealing with Molecular Biology. If you should
ever think about produding a bit further into this field, I highly
recommend this book. It's called: "Molecular Biology" and comes from the -
Instant Notes Series.(Instant notes in Molecular Biology; by Turner,
McLennan, Bates and White)
