For example, why does maroon colored rayon (when bleached) turn into golden yellow? What does this tell you?
Most organic compounds are colorless: white solids or clear, colorless liquids. This is because they don't absorb visible light (they absorb higher-energy ultraviolet light). Dyes are colored because they absorb light in the visible-light region of the spectrum; this is quite different from run-of-the-mill organic compounds.
We need to distinguish dyes, which are chemically bonded to the object being dyed during the "fixing" process, from pigments. Pigments are colored solids that are typically used as a fine powder, and glued to the substrate by being mixed into some sort of binder. That's how paints get their color.
Light, whether ultraviolet or visible, is absorbed by molecules because it promotes electrons to higher energy levels. The energy distance between these levels governs the energy of the light absorbed. Most organic compounds have a large "promotion energy" because even the lowest possible energy jump is rather large. However, conjugation (systems of alternating single and double bonds) lowers the promotion energy. And including some atoms of oxygen or nitrogen in the conjugated system, or perhaps some electrical charge, will lower it even further. Most dyes are conjugated molecules with nitrogen and/or oxygen included.
One interesting example is cyanidine, a natural dye in poppies and cornflowers. The reason poppies are red while cornflowers are blue has to do with the pH of their sap. In acidic poppy sap, all OH groups in cyanidine are protonated and the light absorbed is higher-energy (blue); the leftover light is red. In basic cornflower sap, one of the OH groups is deprotonated and the molecule's absorption shifts to green and red; the leftover light is blue.
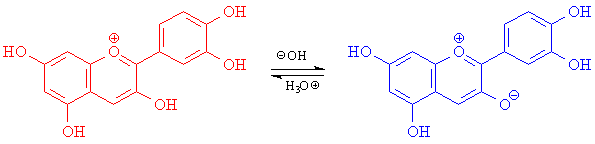
Source: Foxe and Whitesell, Organic Chemistry, 2d Ed., Jones & Bartlett 1997
But when dyes are oxidized (by bleach, for example), the conjugation is disrupted. This disruption of conjugation raises the promotion energy of the dye, and the leftover light shifts toward the blue end of the visible spectrum. (Intense bleaching whitens clothes because it shifts light absorption all the way into the ultraviolet.) One possible reaction of cyanidine is shown below.
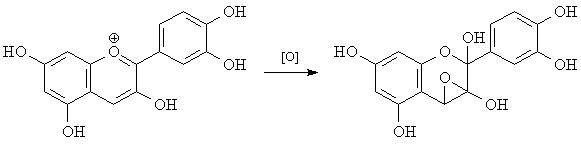
| Dan Berger |
| Bluffton College |
| http://www.bluffton.edu/~bergerd |